 Ravinder received his PhD from National Chiao Tung University (NCTU), Taiwan. He also worked as a Postdoctoral Fellow at National Taiwan University (Taiwan) and University of Waterloo (CANADA). During these eras, his research activities focused on the use of cutting-edge synthetic methodologies and technical skills for the design, efficient synthesis, and characterizations of organic chemical entities (small, supramolecular, and polymers w/and w/o post-polymerizations) for multifaceted applications such as Opto-Electronics, Sensory (Chemo-/Bio-Sensors/OTFTs), Photo-Controllable/Stimuli-Responsive, AIE-based materials (Type-I ROS PSs for Photodynamic therapy and Energy-Transfer), and Metal-Organic Frameworks (MOFs).
Ravinder received his PhD from National Chiao Tung University (NCTU), Taiwan. He also worked as a Postdoctoral Fellow at National Taiwan University (Taiwan) and University of Waterloo (CANADA). During these eras, his research activities focused on the use of cutting-edge synthetic methodologies and technical skills for the design, efficient synthesis, and characterizations of organic chemical entities (small, supramolecular, and polymers w/and w/o post-polymerizations) for multifaceted applications such as Opto-Electronics, Sensory (Chemo-/Bio-Sensors/OTFTs), Photo-Controllable/Stimuli-Responsive, AIE-based materials (Type-I ROS PSs for Photodynamic therapy and Energy-Transfer), and Metal-Organic Frameworks (MOFs).
Prior to this, he acquired 4 years of Pharmaceutical-Industrial Experience; where he started as a Full Time Employee (FTE) at Jubilant Chemsys Ltd., India, an innovator in custom organic synthesis of client-based research. Later, he moved to Ind-Swift Laboratories LTD, India, a leading manufacturer of bulk drugs medicines and pharmaceutical raw materials, where he was promoted to Research Associate-I.
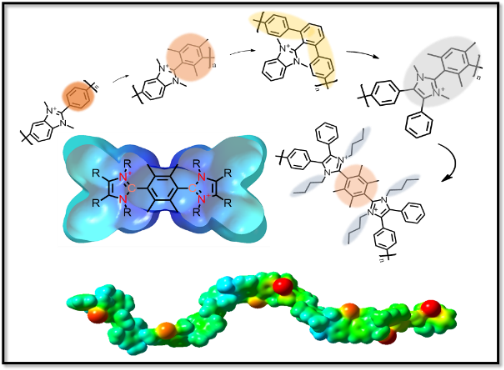
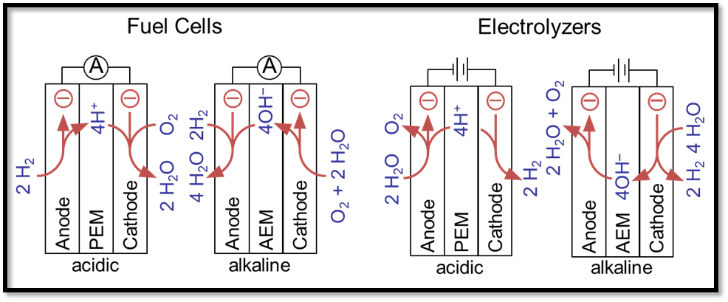
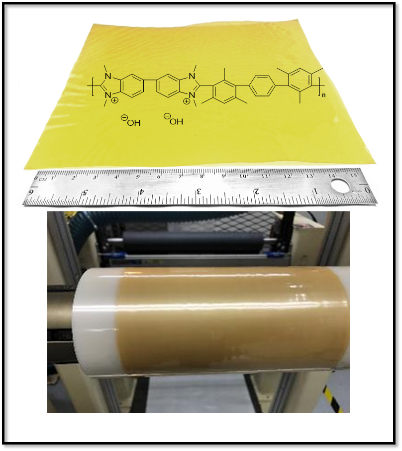
In the Holdcroft lab he is joining the synthetic team currently working on developing numerous N-heterocyclic carbene (NHC)-based polymers for surface-binding applications in anti-corrosion.
In his spare time, he enjoys reading novels, playing cricket, cooking, and exploring nature.























































 Ravinder received his PhD from National Chiao Tung University (NCTU), Taiwan. He also worked as a Postdoctoral Fellow at National Taiwan University (Taiwan) and University of Waterloo (CANADA). During these eras, his research activities focused on the use of cutting-edge synthetic methodologies and technical skills for the design, efficient synthesis, and characterizations of organic chemical entities (small, supramolecular, and polymers w/and w/o post-polymerizations) for multifaceted applications such as Opto-Electronics, Sensory (Chemo-/Bio-Sensors/OTFTs), Photo-Controllable/Stimuli-Responsive, AIE-based materials (Type-I ROS PSs for Photodynamic therapy and Energy-Transfer), and Metal-Organic Frameworks (MOFs).
Ravinder received his PhD from National Chiao Tung University (NCTU), Taiwan. He also worked as a Postdoctoral Fellow at National Taiwan University (Taiwan) and University of Waterloo (CANADA). During these eras, his research activities focused on the use of cutting-edge synthetic methodologies and technical skills for the design, efficient synthesis, and characterizations of organic chemical entities (small, supramolecular, and polymers w/and w/o post-polymerizations) for multifaceted applications such as Opto-Electronics, Sensory (Chemo-/Bio-Sensors/OTFTs), Photo-Controllable/Stimuli-Responsive, AIE-based materials (Type-I ROS PSs for Photodynamic therapy and Energy-Transfer), and Metal-Organic Frameworks (MOFs).

 Lindsay is a proud graduate of Dalhousie University, holding a B. Eng in Chemical Engineering and having completed the enriching co-operative education program. Her journey so far has led her to Rayleigh Solar Tech, a dynamic Halifax-based startup, where she immersed herself in a 16-month endeavor to enhance carbon electrode materials for perovskite solar cells.
Lindsay is a proud graduate of Dalhousie University, holding a B. Eng in Chemical Engineering and having completed the enriching co-operative education program. Her journey so far has led her to Rayleigh Solar Tech, a dynamic Halifax-based startup, where she immersed herself in a 16-month endeavor to enhance carbon electrode materials for perovskite solar cells. Ashley completed her BSc in Chemistry at The King’s University in Edmonton, Alberta. During her degree, she participated in a variety of research, including in vanadium-naphthenic acid coordination in the Athabasca oil sands, computational methods for ab-initio molecular dynamics, and the development of online chemistry education resources.
Ashley completed her BSc in Chemistry at The King’s University in Edmonton, Alberta. During her degree, she participated in a variety of research, including in vanadium-naphthenic acid coordination in the Athabasca oil sands, computational methods for ab-initio molecular dynamics, and the development of online chemistry education resources.
 Apurva earned both her MSc and PhD in Energy Science and Engineering from the Indian Institute of Technology, Bombay. For her Master’s degree, she focused on metal hydride ceramics as hydrogen storage materials, specifically investigating the kinetics of hydrogen sorption.
Apurva earned both her MSc and PhD in Energy Science and Engineering from the Indian Institute of Technology, Bombay. For her Master’s degree, she focused on metal hydride ceramics as hydrogen storage materials, specifically investigating the kinetics of hydrogen sorption.




 Binyu graduated with a B. Eng. in Chemical Engineering from East China University of Science and Technology and a M. Sc in Chemistry from Memorial University of Newfoundland.
Binyu graduated with a B. Eng. in Chemical Engineering from East China University of Science and Technology and a M. Sc in Chemistry from Memorial University of Newfoundland.